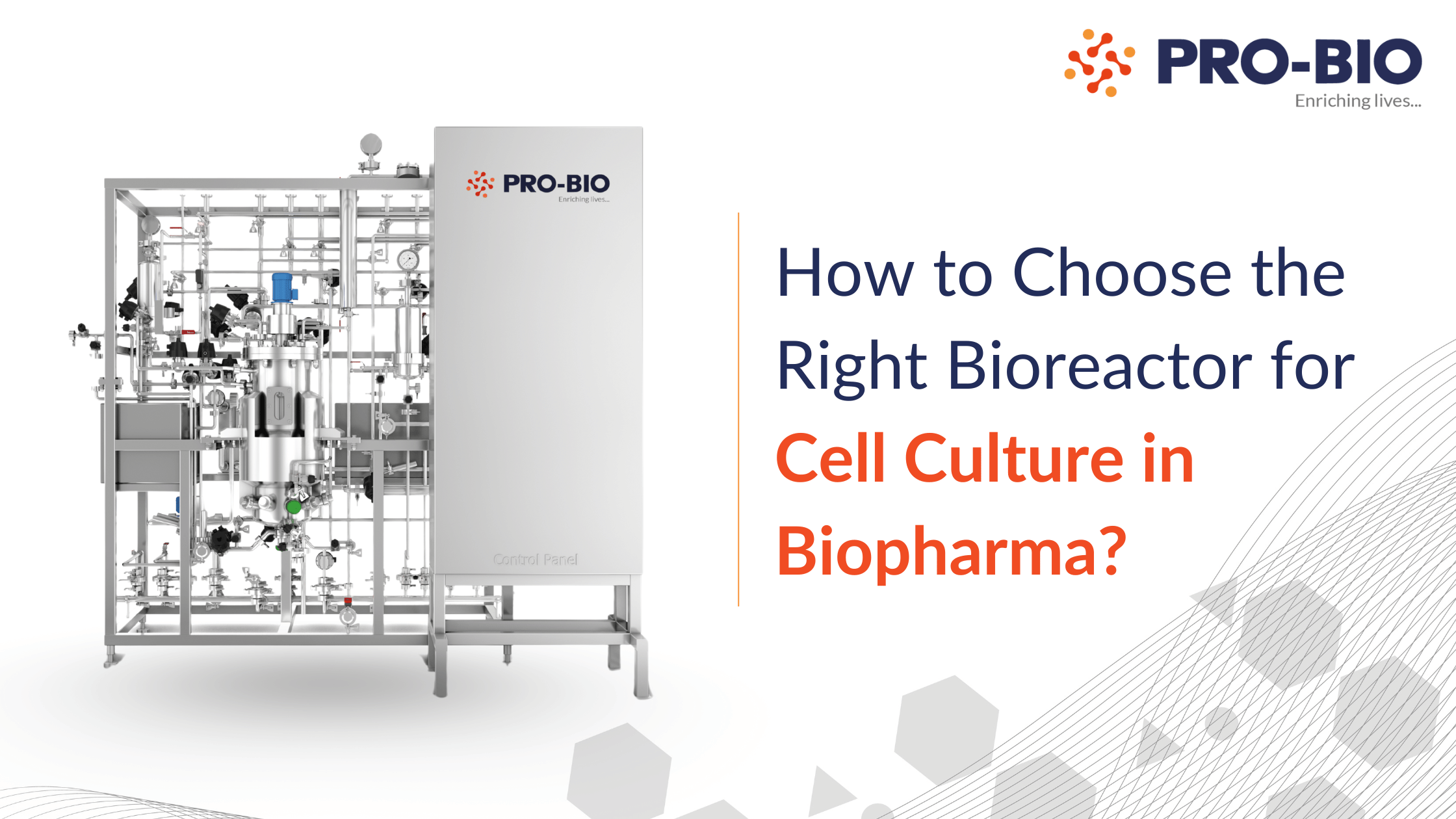
Choosing the right bioreactor for cell culture in a biopharmaceutical manufacturing plant isn’t just about capacity or footprint, it’s about performance, compliance, and process scalability.
With so many configurations available, from lab-scale bioreactors to pilot-scale and GMP production systems, selecting the right platform is essential for biologics developers..
This blog outlines the most important factors to consider when selecting a stainless steel bioreactor for cell culture, especially for companies manufacturing monoclonal antibodies, vaccines, or recombinant proteins.
Why Bioreactor Selection Matters
A bioreactor is the engine of upstream processing. If it fails due to poor oxygen transfer, inconsistent pH, or excess shear you risk a full batch loss.
The right system helps you:
• Maximize cell viability and product yield• Ensure process repeatability from lab to plant
• Simplify validation and audit readiness
• Seamlessly scale into downstream purification
1. Match the Bioreactor to Your Cell Line
Different cells have different needs:
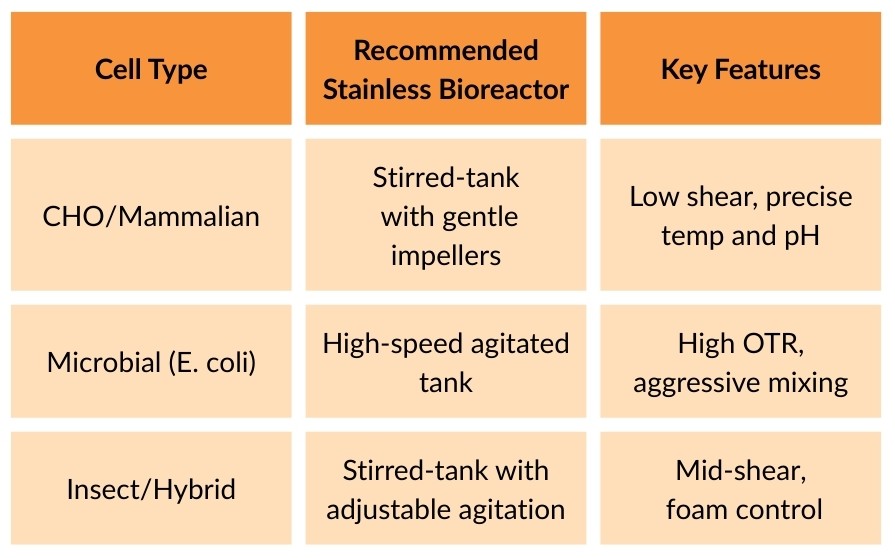
Know your cell line’s oxygen demand, shear sensitivity, and pH tolerance. These define your bioreactor design.
2. Choose the Right Scale: Lab, Pilot, or Production
Bioreactor scale impacts process control and consistency:
• Lab-scale (0.5–10L): R&D and media optimization• Pilot-scale (10–200L): Tech transfer and validation
• Production-scale (500–2,000L+): Full GMP manufacturing
At Pro-Bio, we ensure design consistency across all scales including matching kLa, impeller specs, and sensor configurations to make scale-up frictionless.
3. Why We Recommend Stainless Steel Bioreactors
We specialize in stainless steel bioreactors because they:
• Are robust and ideal for long-term, large-volume use• Support CIP/SIP for automated cleaning and sterilization
• Are fully customizable to your process
• Offer lower cost-per-batch over time compared to single-use
While single-use systems are popular for clinical or niche products, stainless steel is the preferred platform for commercial bio manufacturing, consistency, and regulatory compliance.
4. Control System & Automation
Automation ensures consistency, compliance, and traceability. Look for:
• PLC/SCADA control systems with HMI panels• Recipe-based process control
• 21 CFR Part 11-ready software with audit trails
• Alarm systems, batch logging, and remote monitoring
Pro-Bio’s stainless steel bioreactors include validated automation platforms, designed to support cGMP manufacturing and integration with MES/LIMS.
5. Optimize Agitation and Aeration
Mixing and aeration directly impact cell growth and viability. Key considerations:
• Top or bottom-mounted agitators• Multiple impeller configurations (Rushton, marine, pitched)
• Fine-bubble spargers for optimized gas transfer
• Backpressure and gas blend control for DO precision
Our systems are engineered to deliver consistent kLa and allow fine-tuned oxygen and shear control even at production scale.
6. Compliance, Documentation & Validation
GMP compliance is non-negotiable. Your bioreactor supplier must provide:
• ASME BPE-certified surface finishes• Weld logs, material traceability, and electro polish reports
• FAT/SAT protocols with full IQ/OQ packages
• Automation validation and software compliance
At Pro-Bio, we deliver complete documentation for regulatory readiness, backed by on-site commissioning and training support.
FAQs
Stainless steel stirred-tank bioreactors with low-shear impellers and automated controls are ideal for CHO cells.
Stainless steel is better for commercial-scale, repetitive manufacturing due to its durability and CIP/SIP readiness.
FAT/SAT, IQ/OQ protocols, weld logs, electro polish certificates, and 21 CFR Part 11 software compliance.
Final Thoughts
Choosing a stainless steel bioreactor for your biopharma facility means committing to long-term performance, regulatory success, and scalability.
At Pro-Bio, we specialize in engineering lab to production-scale bioreactors designed for biologics:
• Built to GMP and ASME BPE standards• Fully automated with validated software
• Backed by global service, documentation, and process expertise
Ready to Choose the Right Bioreactor?
📞 Talk to Our Experts📩 Request a Quote
🔎 Explore Our Bioreactor Systems